Over the last few years we have seen an explosion of companies marketing, branding and selling CBD based products. The trend to sell these products in the US will continue to grow as it becomes well know that the Cannabidiol from the cannabis plant does have many medical and health benefits. Many of the CBD based products are sourced from the Hemp plant which makes it easier to sell in the US as most hemp based products can be sold in the US and do not necessarily require FDA approval for sale if marketed as a health supplement and not a medicine.
There are a number of companies growing hemp and then extracting CBD oils for use in bi-products that are then marketed as health supplements by other companies. The supply of CBD has seen tremendous growth over the last few years, the brands and products created and marketed by hundreds of companies does bring a lot of competition to the market. As more people become aware of CBD and its benefits, we do believe the sales of CBD based products will continue to grow. However with more competition these companies will see their profit margins get squeezed and many will fail to bring their products to market.
There are many companies listed on various stock exchanges that participate in product branding and sales of CBD based products. While many have gained attention from the investment community, we do have to stress that most of them are using the same components for their products and it will be difficult to differentiate their products from others. The point is, 90% of these companies do not have anything unique in terms of a product offering and many will fail. If you are going to invest in companies that market and sell CBD based products, make sure they have a great marketing and branding strategy, have strong distribution partners, a proven management team and are well funded.
The main purpose of this article is to get a better understanding of CBD and Cannabidiol, the investment opportunities and value of CBD will be discussed in the future.
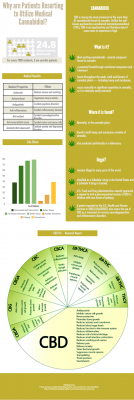
Below is a basic overview of what CBD and Cannabidiol is and the potential health and medical benefits. The exert and infographic below was provided by Monica Bing from http://cannabisowl.com/
Cannabidiol is a major phytocannabinoid and accounts for about 40% of the marijuana plant’s extract. With quite a broad scope of medical applications, it's use is increasing among all populations and countries and research suggests it to be helpful for seizures, anxiety and other ailments.
CBD exerts its actions through a variety of pathways. This non psychoactive component is considered to have a wide scope of potential therapeutic usage. The exact cause for these effects is not clear. But this miracle herb seems to prevent the breakdown of a chemical in the brain that affects pain, mood, and mental function.
CBD is consumed in a variety of forms including oil, tinctures, edibles, capsules, lotion and even chewing gum by patients. But CBD oils are becoming popular among chronic pain sufferers. This oil also works against convulsions, anxiety, insomnia, and ulcers.
In fact, this oil is the new hope of a cure for those suffering from terminal illnesses. Recent studies report that medical use of this oil can help people find relief from severe health conditions like cancer and chemotherapy treatment, atopic dermatitis, epilepsy, HIV/AIDS.
This ultimate oil is also gaining ground as a viable treatment option for several childhood illnesses and conditions. For some parents, CBD for kids becomes a last option. For many families, CBD is a miracle molecule that allows their children to live normal lives without hospitalisations, doctor visits, dangerous medications, and being stuck indoors.
The following infographic outlines the utility of Cannabidiol.